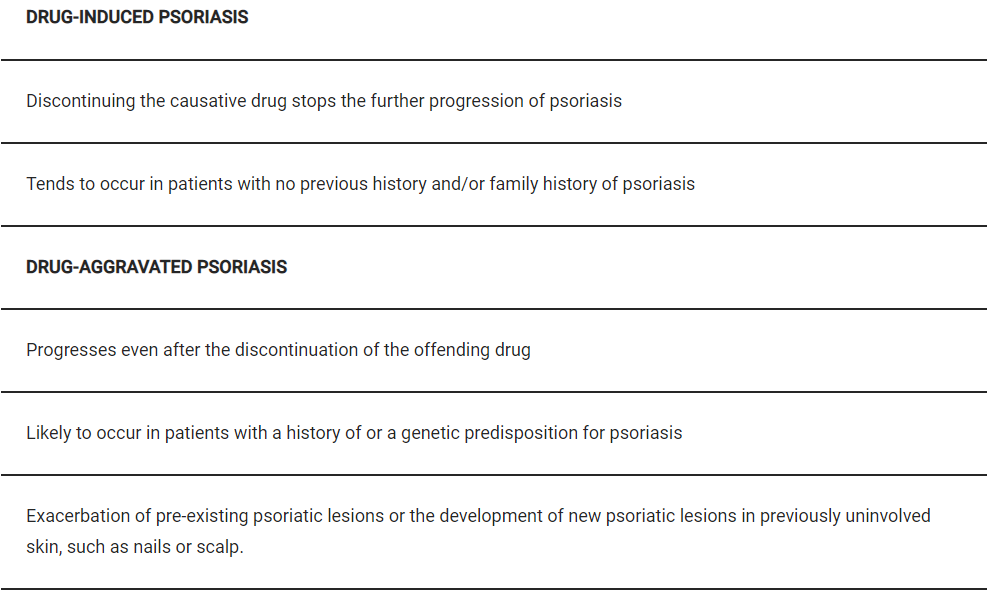
Oral Retinoid Treatment Of Psoriasis – Isotretinoin

Isotretinoin Causes Many Potential Side Effects
Isotretinoin side effects are very common, and sometimes the side effects can be devastating. An Irish man has spent more than 1million Euros taking on a pharmaceutical firm after his son committed suicide. Liam Grant is waging a battle with drugs giant Roche, claiming they are responsible for the death of his son, also called Liam.
In 1996, the 19-year-old started taking Roaccutane, a drug for acne, reports the Independent. He became withdrawn and reclusive and four months later, he hanged himself from a tree outside Dublin.
Since his son’s death, Grant, 61, has shelled out over 1 million fighting the drug’s Swiss manufacturer Roche. He is also pursuing regulators. A scientist hired by him to investigate Roaccutane found – despite studies suggesting a link with depression and suicide – few countries carry warnings on the drug. Roche denies it is to blame for deaths or severe mental health problems.
But Grant can celebrate a significant victory in his fight; he has won a ruling from the European Ombudsman that the European Medicines Agency must release details of all adverse reactions to medications under its rule. The move means for the first time patients will be able to access information on suspected negative reactions across Europe. “I did this out of utter anger,” Grant told the paper. “Roaccutane was licensed for the treatment of acne in 1982. Soon there were studies showing patients got depression within weeks of starting on it. “When I started investigating, I was looking at 20 to 30 published studies linking the drug to depression, psychosis and suicide. Why wasn’t Roche carrying out studies?” But Roche denies responsibility for Liam Grant’s suicide.
“Since 1982, over 15 million patients have been treated with Roaccutane,” said a spokesperson. “Although there have been very rare reports of suicides and suicidal ideation in patients with acne being treated with the medicine, the fact is that severe acne can cause some sufferers to become depressed and can also affect their mood and self esteem.” But the grieving father, whose wife, Loyola, died in 2007, has vowed to fight on. “I am not giving up now,” he added. “I have spent the last 30 years as a forensic accountant investigating some of the biggest frauds in the country and preparing reports for the courts. “That has allowed me to avoid getting involved emotionally and keep objective. It hasn’t dominated my life.”
Eric’s view: And I’m all for it, it’s about time somebody stood up and told pharmaceutical companies the real hard core facts. Noticed how many deaths that hit the media the past few years with regard to the Hollywood people that are pharmaceutical death related? Makes you kind of wonder how many tens of thousands more die you never hear of. I have had bad experiences with Isotretinoin in my clinic with several patients, there are much better ways to treat acne apart from prescribing toxic phramaceutical drugs. Isotretinoin is a synthetic version of Vitamin A. Why not use REAL Vitamin A for acne for goodness sakes?
New Zealanders, like most Westerners are sick to the back teeth of being “fobbed off” by companies such as Roche, just like Mr. Grant has been. Of course they deny responsibility, what is one death when billions of dollars of profits are at stake. The more money they make they more legal clout they have to call the shots and have the most powerful legal defense to defend their lies. Medicine has the nerve to call it “evidence” based medicine, I suppose illness and death related to a pharmaceutical drug is one way of viewing the evidence.
Accutane Lawsuits in 2009/2010 – Ulcerative Colitis
Approximately 5,000 personal injury lawsuits have been filed against Roche Pharmaceuticals, alleging that Accutane caused the onset of bowel problems. Additional lawsuits have been filed against Roche Pharmaceuticals for adverse reactions to Accutane including suicide, psychiatric side effects, and various gastrointestinal disorders.
Accutane Lawsuit 2009: In June of 2009, Roche Pharmaceuticals pulled Accutane from the U.S. market. While the company cited growing competition from generics as a key reason for the pull, Accutane sales had declined dramatically as Roche suffered harsh criticism and a string of lawsuits filed by plaintiffs who claimed the company did not provide proper warning about the dangers of the medication. Interesting fact that Roche ‘no longer make it’, and that in New Zealand Douglas Pharmaceuticals make it. Oh, another interesting fact is that in New Zealand you cannot take a drug company to court. Funny that.
Accutane Lawsuit 2010: In February of 2010, Roche was ordered to pay more than $25 million to a man who developed inflammatory bowel disease years after taking Accutane. The verdict was issued after an earlier decision forcing the company to pay the man $2.5 million was thrown out in a state appeals court.
Read more here: http://www.drugwatch.com/accutane/lawsuit.php
Eric’s Clinical Experiences With Isotretinoin
I do not like this drug and have seen many instances of patients who developed ulcerative colitis and also Crohn’s disease after a history of taking Roaccutane (Isotretinoin). Whenever a patient tells me they took this drug in the past, especially for several years, I begin to wonder if or how it is linked up with the complaints they present with when they see me for a consultation. Do you have psoriasis and take Isotretinoin or Acitretin? Why not take natural Vitamin A instead?
I’ve seen two patients who have complained of persistent dry eyes and/or a very dry tongue for many years after stopping this drug. And naturally, their doctor could see no connection. It’s absolutely amazing how many medical doctors prescribe drugs who have literally no idea about the potential side effects. My advice to you is to very carefully think about a chemical concoction you place in your mouth every day, because it may be causing you real harm, and even an entire new disease may develop that your medical doctor has ‘absolutely no idea’ of its cause.
Here is the information you will find in a doctor’s guide regarding this drug:
ROACCUTANE
Isotretinoin 10mg and 20mg capsules
Retinoid for systemic treatment of acne (but also used for psoriasis by many doctors and dermatologists)
COMPOSITION
Active Ingredient
isotretinoin; 13-cis retinoic acid.
Excipients
Both forms of the capsule contain the excipients soya oil, yellow beeswax, hydrogenated soya bean oil, partially hydrogenated soya bean oil, gelatin, glycerol 85%, sorbitol, mannitol, hydrogenated hydrolysed starch, titanium dioxide, canthaxanthin, shellac (refined) and black iron oxide.
Appearance
Isotretinoin 10mg capsules are reddish violet, opaque, oval, imprinted in black ROA 10, length 9.7mm and diameter 7.0mm.
Isotretinoin 20mg capsules are reddish violet opaque (half) and white opaque (half), oval, imprinted in black ROA 20, length 13.0mm and diameter 8.2mm.
PROPERTIES AND EFFECTS
Mechanism of Action
Isotretinoin, the active ingredient of Roaccutane, is a stereoisomer of all-trans retinoic acid (tretinoin). The exact mechanism of action of Roaccutane has not yet been elucidated in detail, but it has been established that the improvement observed in the clinical picture of severe acne is associated with suppression of sebaceous gland activity and a histologically demonstrated reduction in the size of the sebaceous glands. Furthermore, a dermal anti-inflammatory effect of isotretinoin has been established.
Efficacy
Hypercornification of the epithelial lining of the pilosebaceous unit leads to shedding of corneocytes into the duct and blockage by keratin and excess sebum. This is followed by formation of a comedone and, eventually, inflammatory lesions. Roaccutane inhibits proliferation of sebocytes and appears to act in acne by re-setting the orderly programme of differentiation. Sebum is a major substrate for the growth of Propionibacteriumacnes so that reduced sebum production inhibits bacterial colonisation of the duct.
PHARMACOKINETICS
Since the kinetics of isotretinoin and its metabolites are linear, its plasma concentrations during therapy can be predicted from single dose data. This property also provides some evidence that the activity of hepatic drug metabolising enzymes is not induced by isotretinoin.
Absorption
The absorption of isotretinoin from the gastro-intestinal tract is variable; the absolute bioavailability of isotretinoin has not been determined, since the compound is not available as an intravenous preparation for human use, but extrapolation from dog studies would suggest a fairly low and variable systemic bioavailability. In acne patients at steady state, peak blood concentrations (Cmax) of 310 ng/mL (range: 188-473 ng/mL) were observed 2-4 hours after dosing with 80 mg/day isotretinoin under fasting conditions. Plasma concentrations of isotretinoin are about 1.7 times those of blood concentrations due to poor penetration of isotretinoin into red blood cells.
When isotretinoin is taken with food, the bioavailability is doubled relative to fasting conditions.
Distribution
Isotretinoin is extensively bound to plasma proteins, mainly albumin (≥ 99.9%); therefore the free (= pharmacologically active) fraction of isotretinoin is less than 0.1% over a wide range of therapeutic concentrations.
The volume of distribution of isotretinoin in man has not been determined since isotretinoin is not available as an intravenous preparation for human use.
Steady state blood concentrations (Cmin,ss) of isotretinoin in patients with severe acne treated with 40 mg b.i.d. ranged from 120-200 ng/mL; the concentration of 4-oxo-isotretinoin in these patients was
2-5 times higher than the isotretinoin concentrations. In humans little information is available on the distribution of isotretinoin into tissue. Concentrations of isotretinoin in the epidermis are only half of those in serum.
Metabolism
After oral administration of isotretinoin, three major metabolites have been identified in plasma: 4-oxo-isotretinoin, tretinoin (all-trans retinoic acid), and 4-oxo-tretinoin. The major metabolite is 4-oxo- isotretinoin with plasma concentrations at steady state that are 2.5 times higher than those of the parent compound are. Other minor metabolites have been detected but are not completely identified, which also includes glucuronide conjugates.
Isotretinoin metabolites have shown biological activity in several in-vitro tests. Thus the observed clinical profile in patients could be the result of the pharmacological activity of isotretinoin and its metabolites.
Since isotretinoin and tretinoin (all-trans retinoic acid) are reversibly metabolised (= interconverted), the metabolism of tretinoin is linked with that of isotretinoin. It has been estimated that 20-30% of an isotretinoin dose is metabolised by isomerization.
Enterohepatic circulation may play a significant role in the pharmacokinetics of isotretinoin in man.
In vitro metabolism studies have demonstrated that several CYP enzymes are involved in the metabolism of isotretinoin to 4-oxo-isotretinoin and tretinoin. No single isoform appears to have a predominant role. CYP2C8, CYP2C9, CYP2B6, and possibly CYP3A4 appear to have the greatest contributions in the metabolism of isotretinoin to 4-oxo-isotretinoin. CYP2C9, CYP2B6, and possibly CYP2C8, CYP3A4, CYP2A6, and CYP2E1 contribute to the metabolism of isotretinoin. CYP 26 is also known to metabolize retinoids.
Elimination
After oral administration of radiolabeled isotretinoin approximately equal fractions of the dose were recovered in urine and faeces. Following oral administration of isotretinoin, the terminal elimination half-life of unchanged medicine in patients with acne has a mean value of 19 hours. The terminal elimination half-life of 4-oxo-isotretinoin is longer, with a mean value of 29 hours.
Isotretinoin is a physiological retinoid and endogenous retinoid concentrations are reached within approximately two weeks following the end of Roaccutane therapy.
Pharmacokinetics in Special Populations
Since isotretinoin is contraindicated in patients with hepatic impairment, limited information on the kinetics of isotretinoin is available in this patient population.
INDICATIONS
Severe forms of nodulo-cystic acne which are resistant to therapy, particularly cystic acne and acne conglobata, especially when the lesions involve the trunk.
Roaccutane should only be prescribed by doctors who are experienced in the use of systemic retinoids, preferably dermatologists, and understand the risk of teratogenicity if Roaccutane is used during pregnancy.
DOSAGE AND ADMINISTRATION
Standard Dosage
The therapeutic response to Roaccutane and its adverse events are dose-related and varies between patients. This necessitates individual dosage adjustment during therapy. Roaccutane therapy should be started at a dose of 0.5 mg/kg daily. For most patients the dose ranges from 0.5-1.0 mg/kg per day. Patients with very severe disease or with truncal acne may require higher daily doses up to 2.0 mg/kg.
A cumulative dose of 120 mg/kg per treatment has been documented to increase remission rates and prevent relapse. The therapy duration in individual patients therefore varies as a function of the daily dose. Complete remission of the acne is often achieved by a therapy course of 16-24 weeks. In patients who show severe intolerance to the recommended dose, treatment may be continued at a lower dose with the consequence of a longer therapy duration.
In the majority of patients complete clearing of the acne is obtained with a single treatment course. In case of a definite relapse, a renewed course of Roaccutane therapy should be given with the same daily dose and cumulative treatment dose as previously. Since further improvement of the acne can be observed up to 8 weeks after discontinuation of treatment, retreatment should not be initiated until after this period.
The capsules should be taken with food once or twice daily.
Special Dosage Instruction
Patients with renal impairment
In patients with severe renal insufficiency treatment should be started at a lower dose (e.g. 10 mg/day) and afterwards individually adjusted according to tolerability.
CONTRAINDICATIONS
Roaccutane is contraindicated in:
WARNINGS
It is recommended that clinically significant serum triglyceride elevations be controlled, since levels in excess of 800 mg/dL are sometimes associated with acute pancreatitis, which is known to be potentially fatal (see Undesirable Effects). Hence, Roaccutane should be discontinued if uncontrolled hypertriglyceridaemia or symptoms of pancreatitis occur.
PRECAUTIONS
Roaccutane should only be prescribed by doctors who are experienced in the use of systemic retinoids and understand the risk of teratogenicity associated with isotretinoin therapy (see Pregnancy, Nursing Mothers).
Depression, psychosis and suicidal attempts have been reported with Roaccutane. Particular care needs to be taken in patients with a history of depression, and all patients should be monitored for signs of depression and referred for appropriate treatment if necessary. Although no mechanism of action for these events has been established, discontinuation of therapy may be insufficient and further evaluation by a psychiatrist may be necessary.
Donation of blood by patients should be avoided during and within 1 month after cessation of Roaccutane treatment to prevent an accidental exposure.
Liver function should be checked before and 1 month after the start of treatment, and subsequently at 3 month intervals. Transitory and reversible increases in liver transaminases have been reported. In many cases these changes have been within the normal range and values have returned to baseline levels during treatment. However, when transaminase levels exceed the normal levels, reduction of the dose or discontinuation of treatment may be necessary.
Serum lipids (fasting value) should also be checked, before and one month after the start of therapy, and also at the end of treatment. The serum lipid values usually return to normal on reduction of the dose or discontinuation of treatment. The changes in serum lipids may also resolve in response to dietary measures.
Bone changes, including premature epiphyseal closure, have occurred after several years of administration at high doses for treating disorders of keratinization. Therefore, a careful evaluation of the risk/benefit ratio should be carried out in every patient.
Myalgia and arthralgia may occur and may be associated with reduced tolerance to vigorous exercise (see Undesirable Effects). Isolated instances of raised serum CPK values have been reported in patients receiving Roaccutane, particularly those undertaking vigorous physical activity.
Microdosed progesterone preparations (minipills) may be an inadequate method of contraceptive during Roaccutane therapy.
Aggressive dermabrasion should be avoided in patients on Roaccutane and for a period of 5-6 months after treatment because of the risk of hypertrophic scarring in atypical areas. Wax epilation should be avoided during therapy and at least for a period of 6 months thereafter due to the possibility of scarring or dermatitis.
Decreased night vision has occurred during Roaccutane therapy and in rare instances has persisted after discontinuation of therapy (see Undesirable effects). Because the onset in some patients was sudden, patients should be advised of this potential problem and warned to be cautious when driving or operating any vehicle at night. Visual problems should be carefully monitored.
Dry eyes, corneal opacities, decreased night vision and keratitis usually resolve after discontinuation of therapy. Due to the possible occurrence of keratitis, patients with dry eyes should be monitored. Patients experiencing visual difficulties should be referred for an expert ophthalmological examination and withdrawal of Roaccutane considered.
Rare cases of benign intracranial hypertension (pseudotumor cerebri) have been reported, some of which involved concomitant use of tetracyclines (see Interactions).
Roaccutane has been associated with inflammatory bowel disease (including regional ileitis) in patients without a prior history of intestinal disorders. Patients experiencing severe (haemorrhagic) diarrhoea should discontinue Roaccutane immediately.
Anaphylactic reactions have been rarely reported and only after previous topical exposure to retinoids. Allergic cutaneous reactions are reported infrequently. Serious cases of allergic vasculitis, often with purpura (bruises and red patches) of the extremities and extracutaneous involvement have been reported. Severe allergic reactions necessitate interruption of therapy and careful monitoring.
Precautions for Special Patient Groups
In high risk patients (with diabetes, obesity, alcoholism or lipid metabolism disorder) undergoing treatment with Roaccutane, more frequent checks of serum values for lipids (see Warnings) and/ or blood glucose may be necessary.
In known or suspected diabetics, frequent determination of blood glucose levels is recommended. Elevated fasting blood sugars have been reported, and new cases of diabetes have been diagnosed during Roaccutane therapy.
PREGNANCY, NURSING MOTHERS
Isotretinoin is highly teratogenic. There is an extremely high risk that a deformed infant will result if pregnancy occurs while taking oral isotretinoin in any amount even for short periods. Potentially all exposed foetuses can be affected.
Roaccutaneis contraindicated in women of childbearing potential unless the female patient meets all the following conditions:
Even female patients who normally do not employ contraception because of a history of infertility (except in the case of hysterectomy) or who claim absence of sexual activity must be advised to use effective contraceptive measures while taking isotretinoin, following the above guidelines.
Should pregnancy occur in spite of these precautions during treatment with Roaccutane or in the month following, there is a great risk of very severe malformation of the foetus (involving in particular the central nervous system, the heart and the large blood vessels). There is also an increased risk of spontaneous abortion. If pregnancy does occur, the doctor and patient should discuss the advisability of continuing the pregnancy.
Major human foetal abnormalities related to Roaccutane administration have been documented, including hydrocephalus, microcephalus, abnormalities of the external ear (micropinna, small or absent external auditory canals), microphthalmia, cardiovascular abnormalities, facial dysmorphia, thymus gland abnormalities, parathyroid gland abnormalities and cerebellar malformation.
As isotretinoin is highly lipophilic, the passage of isotretinoin into human milk is very likely. Because of the potential for adverse effects, the use of Roaccutane should be avoided in nursing mothers.
UNDESIRABLE EFFECTS
Most of the side effects of Roaccutane are dose-related. With the recommended dosage, the risk/benefit ratio is generally acceptable considering the severity of the disease.
Symptoms associated with hypervitaminosis A
The following symptoms are the most frequently reported undesirable effects with Roaccutane: dryness of the skin, dryness of the mucosae e.g. of the lips, the nasal mucosa (epistaxis), the pharynx (hoarseness), the eyes (conjunctivitis, reversible corneal opacities and intolerance to contact lenses).
Skin and appendages disorders
Exanthema, pruritus, facial erythema/dermatitis, sweating, pyogenic granuloma, paronychia, nail dystrophy, increased formation of granulation tissue, persistent hair thinning, reversible alopecia, acne fulminans, hirsutism, hyperpigmentation, photosensitivity, photoallergic reactions, skin fragility. Acne flare occurs at the start of treatment and persists for several weeks.
Musculo-skeletal system disorders
Myalgia (muscle pain) with or without elevated serum CPK values (see Precautions), arthralgia (joint pain), hyperostosis, arthritis, calcification of ligaments and tendon and other bone changes, tendinitis.
Psychiatric and central nervous system disorders
Behavioral disorders, depression (see Precautions), headache, increased intracranial pressure (pseudotumor cerebri), seizures.
Sensory disorders
Isolated cases of visual disturbances, photophobia, dark-adaptation disturbances (decreased night vision), rarely colour vision disturbances (reversible upon discontinuation), lenticular cataract, keratitis, impaired hearing at certain frequencies.
Gastro-intestinal system disorders
Nausea, inflammatory bowel disease such as colitis, ileitis, and haemorrhage have been reported to occur. Patients treated with Roaccutane, especially those with high triglyceride levels, are at risk of developing pancreatitis. Fatal pancreatitis has been rarely reported (see Warnings).
Liver and biliary system disorders
Transitory and reversible increases in liver transaminases, some cases of hepatitis. In many such cases the changes have been within the normal range and values have returned to baseline levels during treatment. In other cases, however, it has been necessary to reduce the dose or discontinue treatment with Roaccutane.
Respiratory system disorders
Bronchospasm has been rarely reported; sometimes in patients with a pre-history of asthma.
Disorders of the blood
Decrease in white blood cell count, disorders of red blood cell parameters (such as decrease in red blood cell count and Haematocrit, elevation of sedimentation rate), increase or decrease in platelet count.
Laboratory findings
Increase in serum triglyceride and cholesterol levels, decrease in HDL hyperuricemia. Rare cases of elevated blood glucose have been reported, and new cases of diabetes have been diagnosed (see Precautions).
Resistance mechanism disorders
Local or systemic infections due to Gram positive microorganisms (Staphylococcus aureus).
Miscellaneous reactions
Lymphadenopathy, haematuria, and proteinuria, vasculitis (for example Wegener’s granulomatosis, allergic vasculitis), allergic responses, systemic hypersensitivity, glomerulonephritis.
INTERACTIONS
Concurrent therapy with Roaccutane and vitamin A must be avoided, as symptoms of hypervitaminosis A may be intensified.
Rare cases of benign intracranial hypertension ‘pseudotumor cerebri’ have been reported, some of which involved concomitant use of tetracyclines. Therefore, concomitant treatment with tetracyclines must be avoided (see Precautions).
OVERDOSAGE
Signs of hypervitaminosis A could appear in cases of overdose. Evacuation of the stomach may be indicated in the first few hours after overdosage.
STABILITY
Store below 25°C.
This medicine should not be used after the expiry date shown on the pack.
MEDICINE CLASSIFICATION
Prescription medicine
PACKS
Capsules 10 mg 30’s
Capsules 20 mg 30’s
DATE OF PREPARATION
8 July 2003